Rilpivirine
- Rilpivirine
-
| Rilpivirine |
 |
| Général |
| Nom IUPAC |
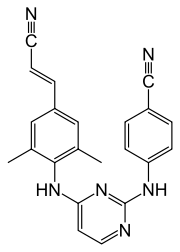
4-{[4-({4-[(E)-2-cyanoéthényl]-2,6-diméthylphényl}amino)pyrimidin-2-yl]amino}benzonitrile |
| No CAS |
500287-72-9 |
| SMILES |
|
| InChI |
InChI : Vue 3D
InChI=1/C22H18N6/c1-15-12-18(4-3-10-23)13-16(2)21(15)27-20-9-11-25-22(28-20)26-19-7-5-17(14-24)6-8-19/h3-9,11-13H,1-2H3,(H2,25,26,27,28)/b4-3+
|
| Propriétés chimiques |
| Formule brute |
C22H18N6 [Isomères]
|
| Masse molaire[1] |
366,4185 ± 0,0201 g·mol-1
C 72,11 %, H 4,95 %, N 22,94 %,
|
|
Unités du SI & CNTP, sauf indication contraire.
|
La rilpivirine (TMC 278) est un médicament antirétroviral à l'étude (mai 2008), c'est un analogue non-nucléosidiques, inhibiteurs de la transcriptase inverse (nNRTI) actuellement développé pour le traitement de l'infection par le VIH.
Ce médicament non encore disponible est attendu en autorisation temporaire d'utilisation (ATU) à défaut éventuellement d'une autorisation de mise sur le marché (AMM) en France.
Efficacité
Elle est comparable à celle de l'éfavirenz et serait mieux tolérée que cette dernière[2],[3].
Voir aussi
Notes et références
- ↑ Masse molaire calculée d’après Atomic weights of the elements 2007 sur www.chem.qmul.ac.uk.
- ↑ Molina J-M, Cahn P, Grinsztejn B, et al, on behalf of the ECHO study group. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial, Lancet, 2011;378:238-246
- ↑ Cohen CJ, Andrade-Villanueva J, Clotet B, et al. on behalf of the THRIVE study group. Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): a phase 3, randomised, non-inferiority trial, Lancet, 2011;378:229-237
Wikimedia Foundation.
2010.
Contenu soumis à la licence CC-BY-SA. Source : Article Rilpivirine de Wikipédia en français (auteurs)
Regardez d'autres dictionnaires:
Rilpivirine — Drugbox IUPAC name = 4 { [4 ({4 [( E ) 2 cyanoethenyl] 2,6 dimethylphenyl} amino)pyrimidin 2 yl] amino}benzonitrile CAS number = 500287 72 9 ATC prefix = ATC suffix = PubChem = 6451164 DrugBank = C=22|H=18|N=6 molecular weight = 366.42 g/mol… … Wikipedia
Emtricitabine/rilpivirine/tenofovir — Combination of Emtricitabine Nucleoside analog reverse transcriptase inhibitor Rilpivirine Non nucleoside reverse transcriptase inhibitor Tenofovir disoproxil fumarate Nucleoside analog reverse transcriptase inhibitor Clinical data … Wikipedia
500287-72-9 — Rilpivirine Rilpivirine Général No CAS … Wikipédia en Français
C22H18N6 — Rilpivirine Rilpivirine Général No CAS … Wikipédia en Français
Discovery and development of non-nucleoside reverse-transcriptase inhibitors — Non nucleoside reverse transcriptase inhibitors (NNRTIs) are antiretroviral drugs used in the treatment of human immunodeficiency virus (HIV). NNRTIs inhibit reverse transcriptase (RT), an enzyme that controls the replication of the genetic… … Wikipedia
Reverse-transcriptase inhibitor — Reverse transcriptase inhibitors (RTIs) are a class of antiretroviral drug used to treat HIV infection, tumors,[1] and cancer.[2] RTIs inhibit activity of reverse transcriptase, a viral DNA polymerase enzyme that retroviruses need to reproduce.… … Wikipedia
Diarylpyrimidines — Chemical structure of etravirine (Intelence) Diarylpyrimidines (DAPY) and diaryltriazines (DATA) are two closely related classes of molecules resembling the pyrimidine nucleotides found in DNA. They show great potency in inhibiting the activity… … Wikipedia
Zidovudine — AZT redirects here. For other uses, see AZT (disambiguation). Zidovudine Systematic (IUPAC) name … Wikipedia
Antiretroviral drug — HAART redirects here. For UK estate agency Haart, see Spicerhaart. Antiretroviral drugs are medications for the treatment of infection by retroviruses, primarily HIV. When several such drugs, typically three or four, are taken in combination, the … Wikipedia
Didanosine — Systematic (IUPAC) name 9 [(2R,5S) 5 (hydroxymethyl)oxolan 2 yl] 6,9 dihydro 3H purin 6 one Clinical data AHFS/Drugs.c … Wikipedia

