Cefpirome
- Cefpirome
-
| Céfpirome |
 |
| Général |
| Nom IUPAC |
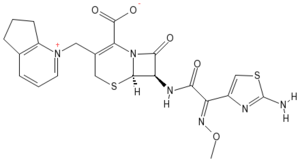
1-{[(6R,7R)-7-[(2E)-2-(2-amino-1,3-thiazol-4-yl)-2-(méthoxyimino)acétamido]-2-carboxylato-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]méthyl}-5H,6H,7H-cyclopenta[b]pyridin-1-ium |
| No CAS |
84957-29-9 |
| Code ATC |
J01DE02 |
| PubChem |
6917674 |
| SMILES |
c12c( ccc[ n+] 1CC1= C( N3C([ C@ H]([ C@ H] 3SC1) NC(= O)\ C(= N\ OC) c1nc( sc1) N)= O) C(= O)[ O-]) CCC2
PubChem, Vue 3D
|
| InChI |
InChI : Vue 3D
InChI= 1/ C22H22N6O5S2/ c1- 33- 26- 15( 13- 10- 35- 22( 23) 24- 13) 18( 29) 25- 16- 19( 30) 28- 17( 21( 31) 32) 12( 9- 34- 20( 16) 28) 8- 27- 7- 3- 5- 11- 4- 2- 6- 14( 11) 27/ h3, 5, 7, 10, 16, 20H, 2, 4, 6, 8- 9H2, 1H3,( H3-, 23, 24, 25, 29, 31, 32)/ b26- 15+/ t16-, 20-/ m1/ s1
|
| Propriétés chimiques |
| Formule brute |
C22H22N6O5S2 [Isomères]
|
| Masse molaire[1] |
514,577 ± 0,032 g·mol-1
C 51,35 %, H 4,31 %, N 16,33 %, O 15,55 %, S 12,46 %,
|
|
Unités du SI & CNTP, sauf indication contraire.
|
La cefpirome est une molécule antibiotique de la classe des céphalosporines de 3e génération.
Mode d'action
La Cefpirome inhibe la PLP, enzyme permettant la synthèse du peptidoglycane bactérien.
Spécialités contenant de la Cefpirome
Notes et références
Wikimedia Foundation.
2010.
Contenu soumis à la licence CC-BY-SA. Source : Article Cefpirome de Wikipédia en français (auteurs)
Regardez d'autres dictionnaires:
Cefpirome — Drugbox IUPAC name = CAS number = ATC prefix = J01 ATC suffix = DE02 PubChem = 6917674 DrugBank = chemical formula = C=22|H=22|N=6|O=5|S=2 molecular weight = 514.58 g/mol bioavailability = protein bound = metabolism = elimination half life =… … Wikipedia
Cephalosporin — See also: Discovery and development of cephalosporins Core structure of the cephalosporins … Wikipedia
Cefquinome — Systematic (IUPAC) name 1 [[(6R,7R) 7 [[(2Z) (2 Amino 4 thiazolyl) (methoxyimino)acetyl]amino] 2 carboxy 8 oxo 5 thia 1 azabicyclo[4.2.0 oct 2 en 3 yl]methyl] 5,6,7,8 tetrahydroquinolinium inner salt … Wikipedia
Ampicillin — Systematic (IUPAC) name (2S,5R,6R) 6 ([(2R) 2 amino 2 phenylace … Wikipedia
Amoxicillin — Systematic (IUPAC) name (2S,5R,6R) 6 … Wikipedia
Penicillin — For the Japanese band, see Penicillin (band). Penicillin core structure. The R is the variable group. Penicillin (sometimes abbreviated PCN or pen) is a group of antibiotics derived from Penicillium fungi.[1] They include … Wikipedia
Beta-lactam antibiotic — β lactam antibiotics are a broad class of antibiotics that include penicillin derivatives, cephalosporins, monobactams, carbapenems, and β lactamase inhibitors, that is, any antibiotic agent that contains a β lactam nucleus in its molecular… … Wikipedia
Timeline of antibiotics — This is the timeline of antimicrobial (anti infective) therapy.The years show when given antibiotic was released onto the pharmaceuticalmarket.* 1910 Arsphenamine aka Salvarsan * 1912 Neosalvarsan * 1935 Prontosil (an oral precursor to… … Wikipedia
Meticillin — Systematic (IUPAC) name (2S,5R,6R) 6 [(2,6 dimethoxybenzoyl)amino] 3,3 dimethyl 7 oxo 4 thia 1 azabicyclo[3.2.0]heptane 2 carboxylic acid Clinical data … Wikipedia
Lysozyme — single crystal Identifiers EC number 3.2.1.17 … Wikipedia

