Ceftizoxime
- Ceftizoxime
-
| Céftizoxime |
 |
| Général |
| No CAS |
68401-81-0 |
| Code ATC |
J01DD07 |
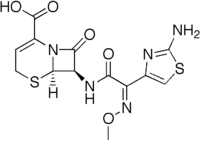
| SMILES |
N12[ C@@ H]([ C@@ H]( NC(\ C( c3csc( n3) N)= N/ OC)= O) C1= O) SCC= C2C( O)= O
PubChem, Vue 3D
|
| InChI |
InChI : Vue 3D
InChI= 1/ C13H13N5O5S2/ c1- 23- 17- 7( 5- 4- 25- 13( 14) 15- 5) 9( 19) 16- 8- 10( 20) 18- 6( 12( 21) 22) 2- 3- 24- 11( 8) 18/ h2, 4, 8, 11H, 3H2, 1H3,( H2, 14, 15)( H, 16, 19)( H, 21, 22)/ b17- 7-/ t8-, 11-/ m1/ s1
InChIKey :
NNULBSISHYWZJU- LLKWHZGFBW
Std. InChI : Vue 3D
InChI= 1S/ C13H13N5O5S2/ c1- 23- 17- 7( 5- 4- 25- 13( 14) 15- 5) 9( 19) 16- 8- 10( 20) 18- 6( 12( 21) 22) 2- 3- 24- 11( 8) 18/ h2, 4, 8, 11H, 3H2, 1H3,( H2, 14, 15)( H, 16, 19)( H, 21, 22)/ b17- 7-/ t8-, 11-/ m1/ s1
Std. InChIKey :
NNULBSISHYWZJU- LLKWHZGFSA- N
|
| Propriétés chimiques |
| Formule brute |
C13H13N5O5S2 [Isomères]
|
| Masse molaire[1] |
383,403 ± 0,024 g·mol-1
C 40,72 %, H 3,42 %, N 18,27 %, O 20,86 %, S 16,73 %,
|
|
Unités du SI & CNTP, sauf indication contraire.
|
La ceftizoxime est une molécule antibiotique, une céphalosporine de 3ème génération.
Mode d'action
La céftizoxime inhibe la PLP, enzyme permettant la synthèse du peptidoglycane bactérien.
Notes et références
Wikimedia Foundation.
2010.
Contenu soumis à la licence CC-BY-SA. Source : Article Ceftizoxime de Wikipédia en français (auteurs)
Regardez d'autres dictionnaires:
Ceftizoxime — Systematic (IUPAC) name (6R,7R) 7 {[(2Z) 2 (2 amino 1,3 thiazol 4 yl) 2 methoxyiminoacetyl]amino] 8 oxo 5 thia 1 azabicyclo[4.2.0]oct 2 ene 2 carboxylic acid Clinical data … Wikipedia
Ceftizoxime — См. Цефтизоксим (Источник: «Словарь терминов микробиологии») … Словарь микробиологии
ceftizoxime — noun A parenteral third generation cephalosporin drug … Wiktionary
ceftizoxime sodium — A broad spectrum cephalosporin antibiotic similar to cefotaxime sodium. * * * cef·ti·zox·ime so·di·um (sef″tĭ zokґsēm) [USP] a semisynthetic, β lactamase–resistant, third generation cephalosporin effective against a wide… … Medical dictionary
Cephalosporin — See also: Discovery and development of cephalosporins Core structure of the cephalosporins … Wikipedia
Ampicillin — Systematic (IUPAC) name (2S,5R,6R) 6 ([(2R) 2 amino 2 phenylace … Wikipedia
Amoxicillin — Systematic (IUPAC) name (2S,5R,6R) 6 … Wikipedia
Beta-lactamase — Beta lactamases are enzymes (EC number|3.5.2.6) produced by some bacteria and are responsible for their resistance to beta lactam antibiotics like penicillins, cephalosporins, cephamycins, ertapenems and carbapenems. These antibiotics have a… … Wikipedia
Penicillin — For the Japanese band, see Penicillin (band). Penicillin core structure. The R is the variable group. Penicillin (sometimes abbreviated PCN or pen) is a group of antibiotics derived from Penicillium fungi.[1] They include … Wikipedia
Meticillin — Systematic (IUPAC) name (2S,5R,6R) 6 [(2,6 dimethoxybenzoyl)amino] 3,3 dimethyl 7 oxo 4 thia 1 azabicyclo[3.2.0]heptane 2 carboxylic acid Clinical data … Wikipedia

